Pfizer's legal department navigates the challenges of bringing COVID vaccine to market in Canada
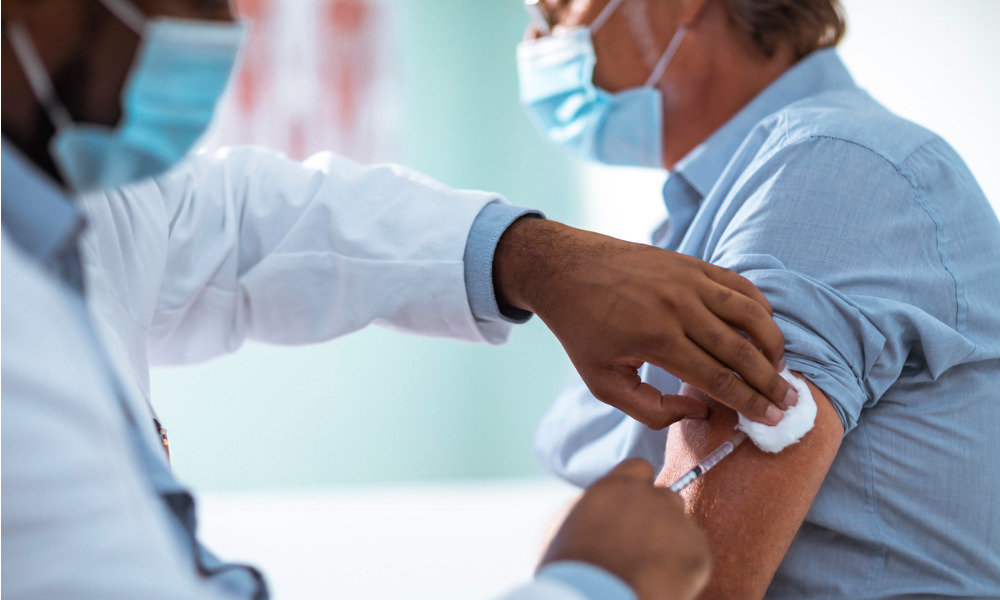
As the world continues to battle a health- and economy-crushing pandemic, Canadian medical and pharmaceutical companies are working around the clock to deliver life-saving medications safely around the country, and some players are even developing critical vaccines.
“This is a huge opportunity for the life sciences industry,” says Vanessa Grant, a partner at Norton Rose Fulbright Canada LLP. “A spotlight has been shone on it that we may never see again, as more people than ever are turning to life sciences, and that reflects the industry’s traditional resilience in the face of economic challenges.”
While the world leans heavily on this powerful industry, the globally integrated supply chain will continue to present problems for life sciences companies while hospitals scramble to clear a vast backlog of surgeries, according to Grant.
“One of the challenges but also an opportunity that I see for our clients in the industry is the expansion of domestic production capacity for pharmaceuticals, medical devices and personal protective equipment,” she says. “I think the real challenge will continue to be to supply sufficient products.” Supply and demand issues around the new vaccine will be a major challenge for the industry, together with communication of any issues that arise, Grant says.
Pfizer is at the forefront of new developments as the global pharmaceutical giant is the first manufacturer to begin rolling out a vaccine for COVID-19 that was developed and tested at unprecedented speed, in partnership with Germany’s BioNTech.
“The interesting thing for us has really been the increase in the pace of activities because of our critical role during the pandemic crisis this year,” says Karine Grand’Maison, senior legal counsel at Pfizer. “Between helping young children with their virtual classes and ordering online groceries for our parents, we can’t drop the ball on our purpose.”
Continuing to meet the company’s primary purpose of bringing therapies and medications to patients in need during the crisis was a key responsibility for Grand’Maison and her team. Pfizer’s legal team played an essential role in supporting the health and well-being of employees and in navigating interactions with health-care professionals and stakeholders during a year of extreme uncertainty and rapidly changing regulations.
Supporting the rollout of Pfizer and BioNTech’s vaccine — which was approved for use in Canada in December — is the latest focus of the legal team, which raises a host of new challenges in an already highly regulated industry.
“In the legal team, we are really helping the organization manage the interrelation between us as manufacturers and the governments, health-care professionals and patients who need our medicines,” says Grand’Maison. “Everything related to bringing the COVID-19 vaccine to market is extraordinary. From submitting an application for authorization, providing information to health-care professionals and managing IP rights to supporting the vaccine rollout, everything is extraordinary, given the importance of the pandemic and the high level of public interest.”
Pfizer Canada is making every effort to ensure that the approach to launching the vaccine in Canada is very much aligned with the company’s global response. Another strategy being adopted by the legal team is to follow a principle-based approach to providing legal advice.
“The principle-based approach enables us to provide legal and ethical principles to our commercial and cross-functional colleagues, so they are empowered to make quick, timely decisions within the parameters that are provided,” says Grand’Maison. “This way, we can work with incredible speed and bring our vaccine candidate to market as soon as possible with the highest standards.”
Grand’Maison and her team are committed to ensuring that they work closely with regulatory agencies and all stakeholders that play a role in the launch of the vaccine, as well as monitoring risks so that nothing is overlooked that could create problems in the future.
“It has been a privilege for me to be working at a company that is part of the worldwide efforts to help protect the health of everyone around us,” says Grand’Maison. “Given the great collaboration between the industry and all stakeholders, I hope that Canadians will be able to get back to a new normal in their everyday lives.”
Compliance is a critical concern for all companies involved in the manufacturing and distribution of COVID vaccines.
“Whenever there is something new with limited access, there’s always someone who wants to jump the line, so we need to make sure we are complying with the laws in terms of access,” says Cheryl Reicin, a partner and chairwoman of the life sciences practice at Torys LLP. “The whole world is focused on this industry. I suspect there will be some missteps here in the whole access line so that should really be the main focus, to make sure there is really good compliance — both in terms of storage and in terms of access, because there will be a big backlash if companies don’t comply.”
Grant is optimistic that the life sciences industry will continue to flourish and that strong fundamentals will drive mergers and acquisitions within the industry in 2021.
“We believe the outlook is bright from a transactional perspective, a regulatory perspective and from a research and development perspective,” she says. “It has been a huge opportunity and the lessons learned in this pandemic will benefit research and development moving forward.”










